Auditory Brainstem Implant

What is an auditory brainstem implant (ABI)?
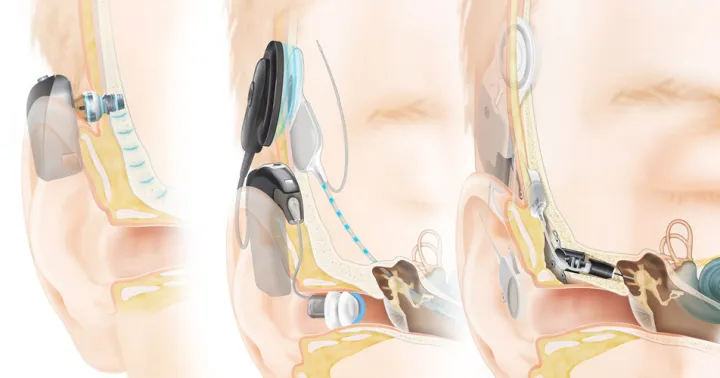
Cochlear implants work by stimulating the remaining auditory nerve fibers. However, if a disease or tumor removal leaves the auditory nerve unable to function, the patient may not be a candidate for a cochlear implant. The Auditory brainstem implant (ABI) was developed to restore hearing to patients in this situation. In most cases, these are patients with neurofibromatosis type 2 (NF2) which means they have tumors in both ears. As a result of the tumors or their surgical removal, hearing is lost and the auditory nerves are destroyed. The ABI bypasses the damaged auditory nerves by having electrodes placed in the highest centers of the central auditory pathway close to the brain which is the cochlear nucleus in the brainstem.
Generally, the ABI is implanted during the acoustic neuroma resection. It can be placed during the removal of the first of two tumors, which gives the patient time to adjust before complete loss of hearing when a second surgery removes the tumor at the other ear. The tumor is removed via the translabyrinthine approach, and as soon as the ABI is implanted, auditory electrical response tests are performed. These tests help determine whether the patient will be able to hear the sounds generated by this device.
Overall, the hearing of patients who received ABIs is generally similar when compared to patients who received conventional cochlear implants. 85% of patients who receive an ABI have auditory sensation. The ABI can be used in children as well as adults who do not have NF2 but who have sensorineural hearing loss and are not candidates cochlear implants due to absence of the cochlear nerve, absence of the cochlea, ossification due to meningitis, trauma, or severe otosclerosis.

